
Aluminium oxide appears in nature as α-Al2O3 (=Corundum). The hexagonal α-Al2O3 is the only thermodynamically stable modification. Besides there is the cubic face-centered γ-Al2O3, which develops from aluminium hydroxide at 400-800°C (752-1472°F) and is finaly converted to α-Al2O3 at 1100°C (2013°F).
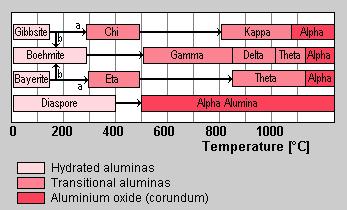
Dehydration sequence of hydrated alumina in air. Path (b) is favoured by moisture, alkalinity, and coarse particle size (100µm), path (a) by fine crystal size (<10µm). (after Gitzen, Walter H.; Alumina as a Cermaic Material, The American Ceramic Society. Inc., Columbus Ohio.)
Technically corundum is manufactured today by electric melting of bauxite, which is the starting material for refractory products. Thus, for example crucibles, protective tubes and refractory bricks are produced as sintered corundum by pressing α-Al2O3 with bonding agents such as clays, which are fired at approx 1300°C (2372°F). Alumina coatings are available as Aluminium-Oxide-Coating AA and Aluminium-Oxide-Coating AW.
Properties of aluminium oxide |
|
Formular |
Al2O3 |
Molecular mass |
101.96 g/mol |
Density |
3,98 g/cm³ |
Hardness |
9 |
Melting point |
2053°C (3727°F) |
CAS-No. |
1344-28-1 |
EINECS-No. |
215-691-6 |
© 2023 Büro für angewandte Mineralogie · Dr. Stephan Rudolph · D-47918 Tönisvorst
These recommendations are believed to be correct. However, no guarantee of their accuracy is given. Therefore, purchasers shall make their own tests to determine suitability for their use. These products are offered for industrial and related uses (e.g. research and development) only. However the user must take the necessary precautions appropriate for products containing chemicals. This description does not imply the absence of any patents, the responsibility whatsoever solely rests with the user.
www.a-m.de